Our strategy is built around three vectors that are rapidly changing the field of possibility.
Actionable Genetics
The biological blueprint of disease probability.
Brain Circuitry
The brain’s roadmap for pathological decision-making.
Precision Therapies
Targeted treatments that meet the needs of the individual.
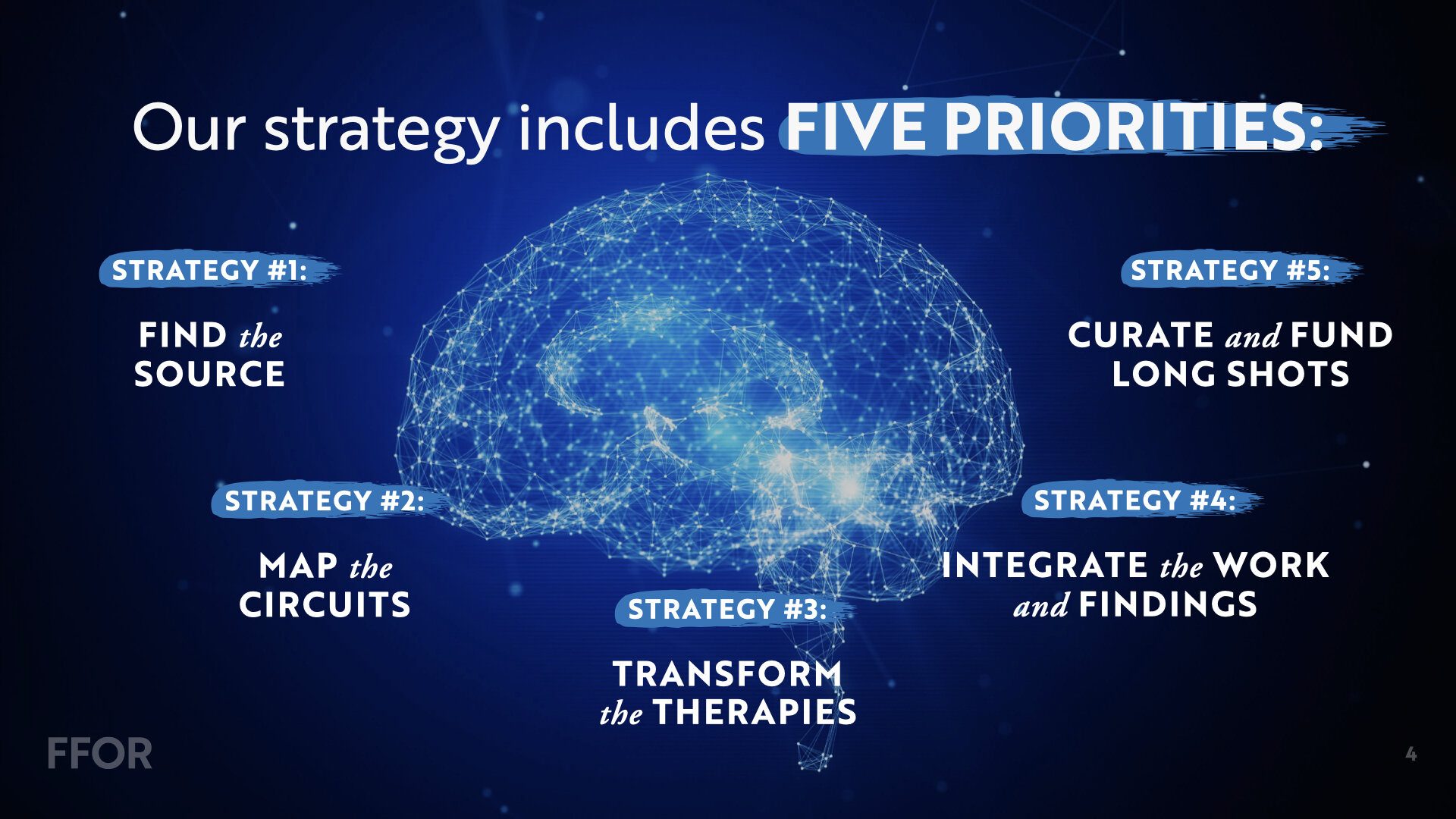
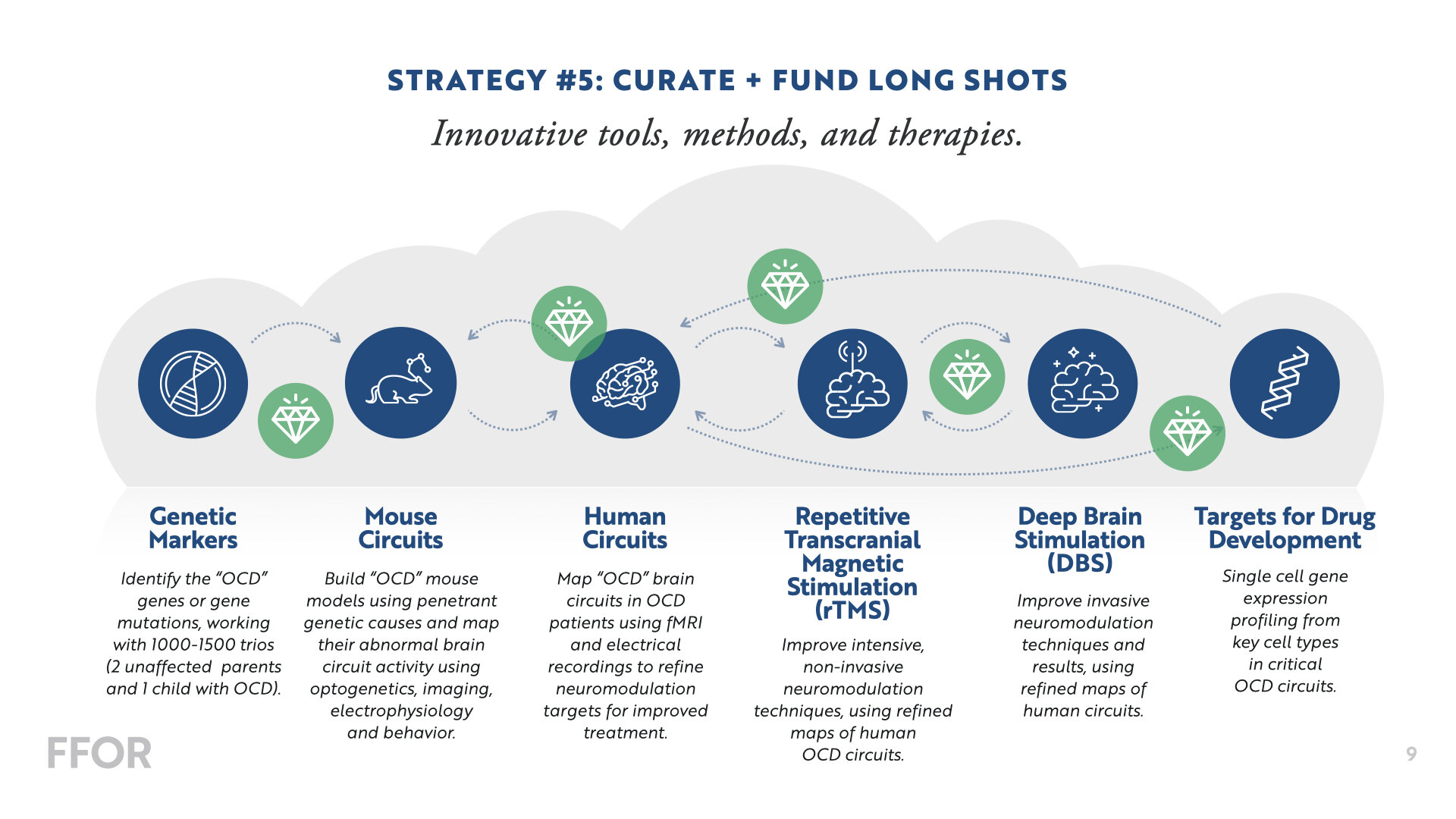
Our strategy includes five priorities that will drive the future of OCD treatment + discovery.

Overview of funded FFOR projects
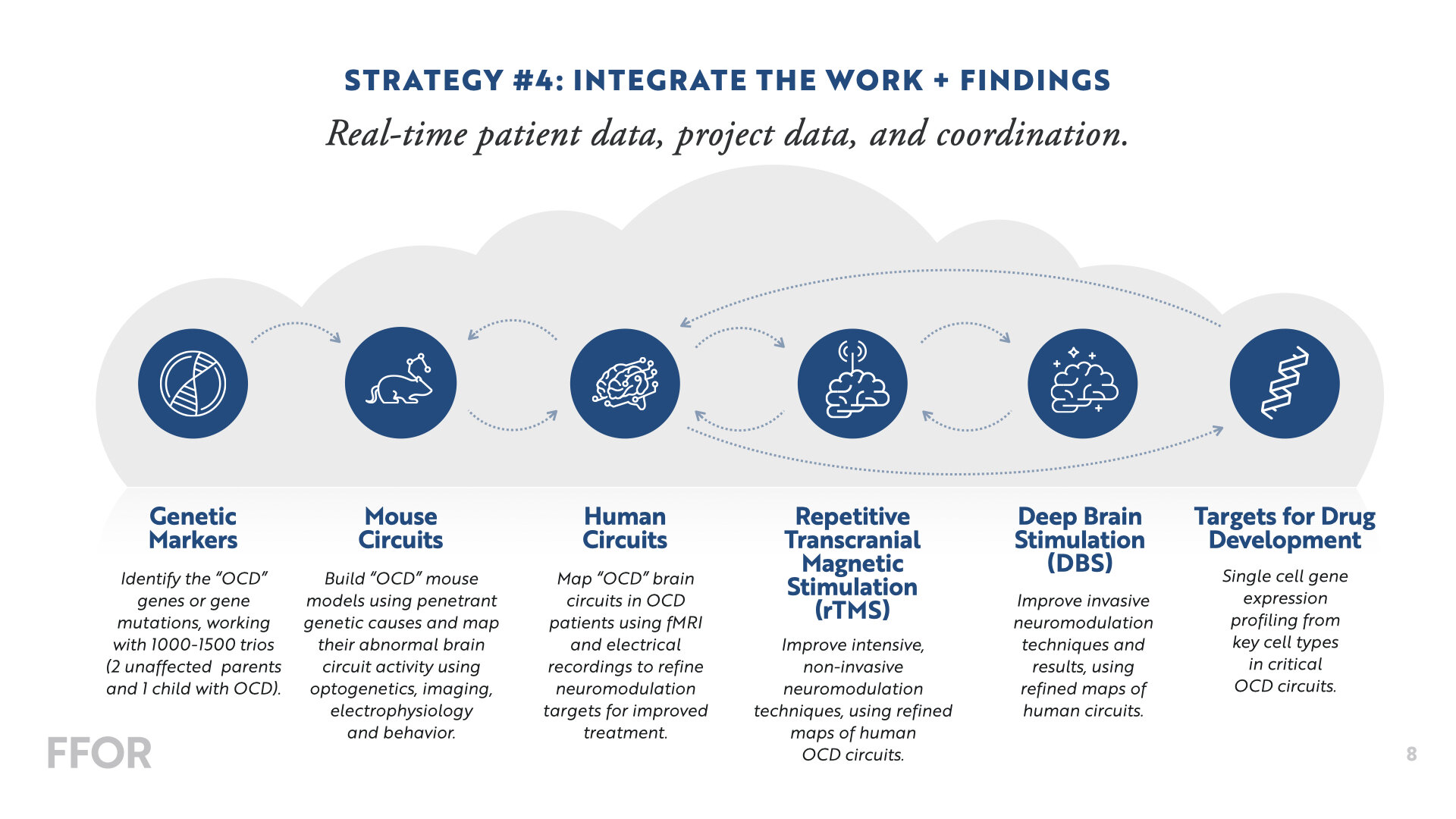
We’re making big investments in a core team of Principal Investigators to collaborate on several multi-year, multi-disciplinary projects in the areas of Genetics, Neuroscience, and Therapeutics—with the goal of developing a “functional” cure. Below is a snapshot of our current projects.
Breaking through in OCD genetics
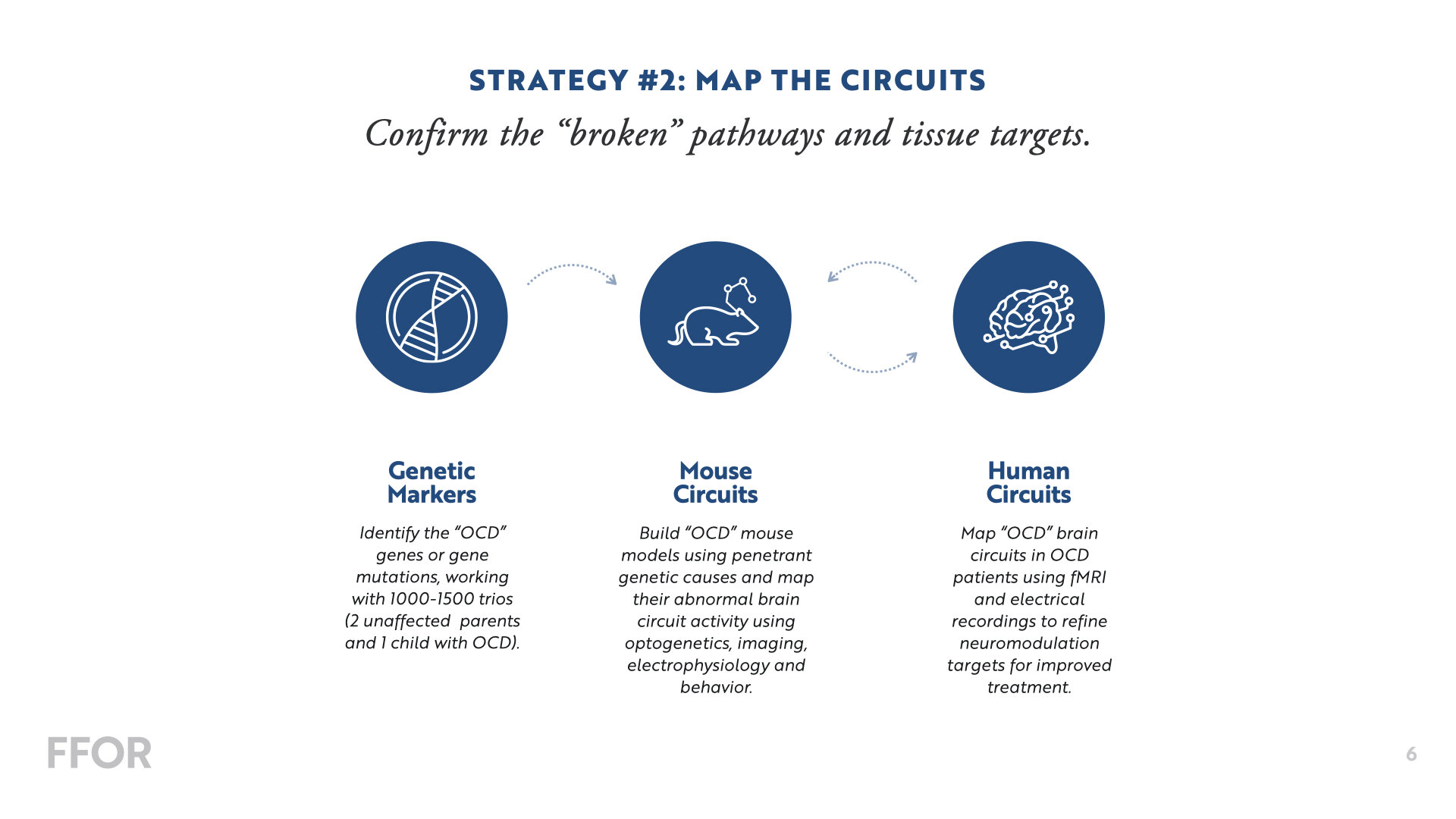
It is difficult to characterize the results to date of the search for genes underlying obsessive compulsive disorder (OCD) as anything but disappointing. However, recent advances by our group and others have set the stage to reverse this trend dramatically and rapidly by reliably identifying large-effect risk genes. These risk genes will provide essential molecular clues to the origins of OCD and offer important avenues for the development of new and more successful treatments.
The approach we propose to take for OCD mirrors methods that have been applied with great success to a wide range of neurodevelopmental disorders, including autism spectrum disorder, Tourette disorder, epilepsy, intellectual disability, and ADHD. The specifics of this approach are now well worked out: we will ascertain families with simplex OCD (unaffected parents and only one child diagnosed with OCD) and then identify rare and spontaneous (de novo) mutations (meaning the genetic mutation was not inherited from the parents). We will then leverage these mutations to identify large-effect risk genes, providing a direct avenue for FFOR investigators and others to illuminate pathophysiology.
GOAL 1: Recruit 1,000 well-characterized OCD simplex trios.
GOAL 2: Identify de novo mutations in the coding portion of the genome and leverage them to discover OCD risk genes.
GOAL 3: Identify de novo copy number variants (large deletions or duplications of DNA) and pinpoint additional genomic regions that carry risk.
GOAL 4: Share these data with other groups in order to bolster gene discovery in OCD.
Targeting Cell Types and Circuits to Treat OCD
OCD has lagged behind other neuropsychiatric disorders in discovery of new treatments due to lack of clear molecular candidates for new drug development and lack of sufficient understanding of the malfunctioning neural circuits. FFOR is now attacking this problem directly by discovering de novo mutations of large effect size. While these new targets are being identified, the FFOR Mouse Circuit Consortium is identifying the neural circuits and cellular components that underlie OCD-relevant behaviors, so that we can rapidly deploy these new genetic discoveries into the circuits and cell types most relevant to processes disrupted in OCD. Although insights regarding dysfunctional circuits in OCD have been obtained via human neuroimaging studies, they are inherently limited due to lack of cellular resolution and inability to directly test cause and effect.
Recent technological advances now allow us to directly monitor and manipulate specific cell types and circuits in mice to test causality and mechanism, providing a crucial bridge between neural functions relevant to human symptoms and genes. These mechanistic studies will ultimately allow us to determine how identified genetic mutations alter function in key circuits, and pave the way towards development of novel circuit-specific pharmacological or neural stimulation approaches.
The FFOR Mouse Circuit Consortium has 3 overarching goals to support this endeavor by coordinating and integrating research efforts across our four sites.
GOAL 1: Dissect circuits underlying key neural functions relevant to OCD pathology and treatment.
GOAL 2: Identify cell-types crucial to OCD pathology and treatment.
GOAL 3: Perform preclinical studies to facilitate the work of the FFOR rTMS and DBS research groups.
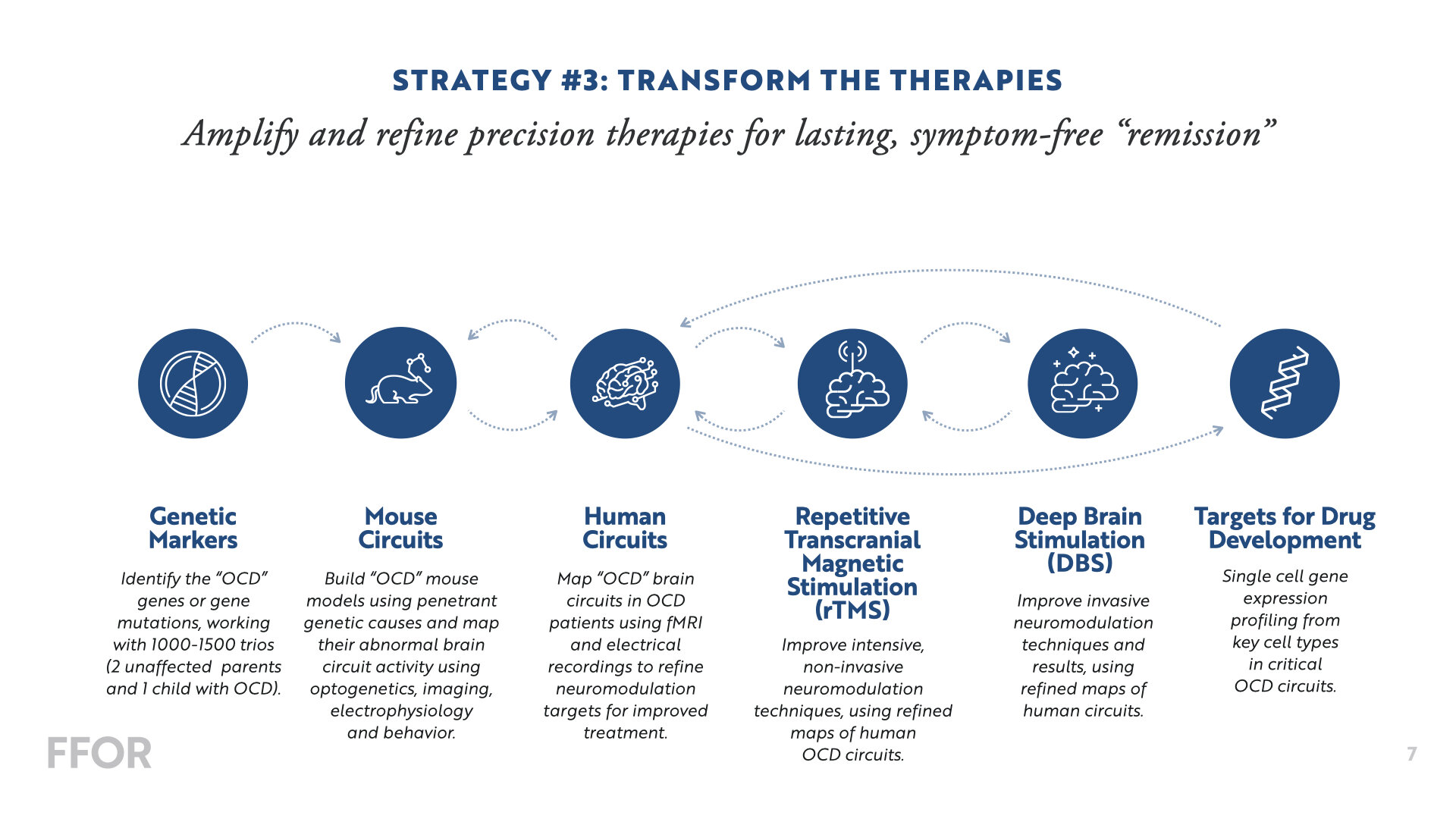
Accelerated Transcranial Magnetic Stimulation: Optimizing a New Treatment for OCD
Obsessive compulsive disorder (OCD) is a common neuropsychiatric condition that affects approximately 2% of the population and is often highly debilitating. Unlike many diagnoses in other areas of medicine, OCD is not a single disease: it is expressed differently from person to person, and it is likely that there are multiple forms of OCD caused by distinct disease processes. Medications and therapy are the standard treatments; however, many patients show only a partial improvement, and more than 25% of patients show no improvement with these treatments. New interventions are urgently needed. Repetitive transcranial magnetic stimulation (rTMS) is a promising alternative, using powerful, focused magnetic pulses to stimulate two brain areas, the dorsomedial prefrontal cortex and the orbitofrontal cortex. Patients often show a strong response to stimulating one target but not the other. It is not well understood why some patients respond, while others do not. Recent evidence suggests that an accelerated course of 10x daily rTMS for five days not only accelerates treatment response but also yields markedly higher remission rates in depression, and the same principle may apply in cases of OCD. Our project will optimize and test a new accelerated rTMS protocol for treating OCD. In parallel, we will apply methods we have developed to discover circuit subtypes of OCD and develop prognostic neuroimaging biomarkers for predicting differential treatment response to rTMS targeting the orbitofrontal or dorsomedial prefrontal cortex.
Dissection of circuit-typed OCD to optimize a next generation deep brain stimulation strategy
Deep Brain Stimulation (DBS) can provide symptomatic relief in many OCD patients refractory to conventional medical and cognitive therapies. However, as many as 40% of patients do not respond to this form of invasive neuromodulation, and most patients will still have significant residual symptoms.
The lack of consistent benefit with existing forms of DBS for OCD is likely multi-factorial, due to:
heterogeneity in the indication
suboptimal targeting of relevant circuits
lack of sufficient target engagement with conventional stimulation protocols
Here, we aim to overcome these barriers by collaborating with our FFOR partners to identify circuit-defined OCD biotypes responsive to neuromodulation, i.e. rTMS. We will then utilize a novel approach to utilizing stereoencephalography (sEEG) to map neural networks underlying OCD symptoms and identify potentially novel DBS targets. These studies will then guide the development of computationally informed stimulation protocols that can be incorporated into next-generation neurostimulation devices. A multidisciplinary team of neurophysiologists, data scientists, psychiatrists, and neurosurgeons based at Stanford and UCSF will be leading this project in collaboration with cognitive researchers at UCLA and computational neuroscientists at Mayo Clinic. The study will culminate in a randomized control early feasibility trial of our novel DBS approach for treating refractory OCD.



